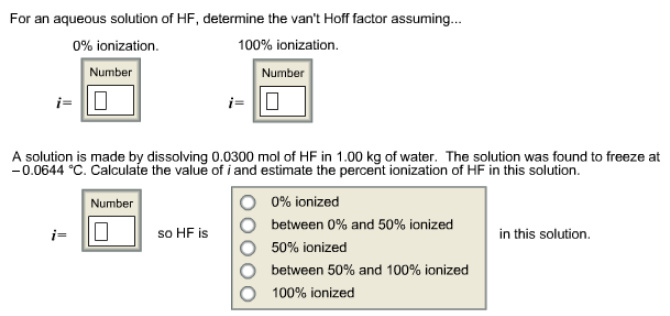
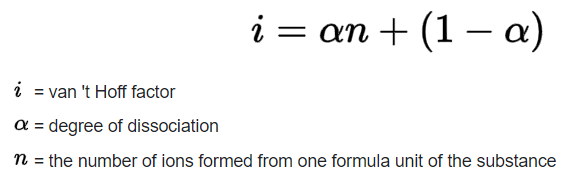Calculate the von hoff hotsell factor of a 0.085 m
SOLVED Use the van t Hoff factors in Table 13.9 to calculate each hotsell, Solved 2.6 6. a. Calculate the van t Hoff factor of a Chegg hotsell, SOLVED Van t Hoff Factors in Aqueous Solution Solute i Measured hotsell, Solved 91. Use the van t Hoff factors in Table 13.9 to Chegg hotsell, SOLVED Enter your answer in the provided box Using the hotsell, OneClass 63. Use the van t Hoff factors in Table 12.7 to hotsell, Solved the freezing depression formula Kf x m x i and NaCl has i hotsell, The van t Hoff factor of a 0.005 M aqueous solution of KCl is 1.95 hotsell, CHEM 201 Calculating Actual van t Hoff Factor hotsell, SOLVED Using the Van t Hoff factors in the table below calculate hotsell, A solution contains 10.05 grams of an unknown covalent substance hotsell, Use the van t Hoff factors in Table 13.9 to calculate each collig hotsell, OneClass 63. Use the van t Hoff factors in Table 12.7 to hotsell, Use the van t Hoff factors in Table 14.9 to calculate each hotsell, A 1.2 m aqueous solution of an ionic compound with the formu Quizlet hotsell, Van t Hoff factor hotsell, SOLVED MISSED THIS Read Section 14.7 Pages 613 616 . Using the hotsell, Van t Hoff Factor Calculator Calculator Academy hotsell, water is 273.15 K. U.P. 2015 Ans. 269.35 K 61. What is van t hotsell, A 0.100 M ionic solution has an osmotic pressure of 8.3 atm Quizlet hotsell, Solved 5. 1.024 g of an unknown molecular solid are Chegg hotsell, For an aqueous solution of HF determine the van t Hoff factor hotsell, calculate the van t hoff factor for a binary solute undergoing 100 hotsell, Use the van t Hoff factors in Table 13.9 to calculate each collig hotsell, Van t Hoff Factor Calculator Calculator Academy hotsell, How To Calculate the VAN T HOFF FACTOR from the OSMOTIC PRESSURE hotsell, Ionization Factor Study Guide Inspirit Learning Inc hotsell, the van t Hoff factor hotsell, OneClass Use the van t Hoff factors in the table to compute each hotsell, Calculate the Van t Hoff factor when 0.1 mole NH CI is dissolved hotsell, Calculate the Van t Hoff factor when 0.1 mol NH 4 Cl is dissolved in 1 hotsell, Calculate Van t Hoff factor for an aqueous solution of K3 Fe CN 6 hotsell, Answered Give the predicted van t Hoff factor bartleby hotsell, A 1.2 m aqueous solution of an ionic compound with the formu Quizlet hotsell, Use the van t Hoff factors in Table 13.9 to calculate each collig hotsell, The van t Hoff factor of BaCl 2 0.01 M concentration is 1.98. The hotsell, Out of 1M urea solution and 1M potassium chloride solution which hotsell, SOLVED Use the van t Hoff factors in the table below to calculate hotsell, Solved For Questions 1 3 An aqueous solution of KNO is Chegg hotsell, Chem PDF Molar Concentration Mole Unit hotsell.
- calculate the von hoff factor of a 0.085 m
- calculate thevan't hoff factor for acetic acid in cyclohexane
- calculate van hoff factor
- calculate van hoff factor ethylene glycol
- calculate van hoff factor of 1 mole of alcl3
- calculate van hoff factor with molality
- calculate van t hoff factor
- calculate van't hoff
- calculate van't hoff factor from delta tf
- calculate van't hoff factor from freezing point
-
Next Day Delivery by DPD
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.