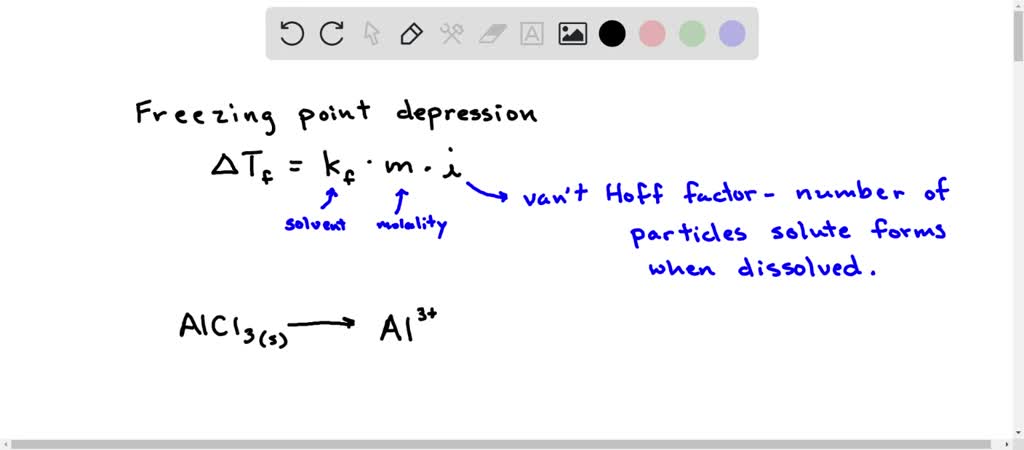
Freezing point depression vant hoff hotsell factor of nacl in water
Freezing Point Depression Formula and Definition hotsell, Predicting Relative Freezing Point Depressions Chemistry Study hotsell, SOLVED The freezing point depression constant Kf is 1.86 hotsell, SOLVED You have an aqueous solution of NaCl that has a freezing hotsell, The depression in freezing point is 0.24K obtained by dissolving hotsell, Solved 14 Question 5 points See page 570 The freezing Chegg hotsell, SOLVED Information on the Extent of Freezing Point Depression hotsell, Solved 1.86 Freezing point depression constant C m Chegg hotsell, what mass of NaCl must be dissolved in 65 g of water to lower the hotsell, Boiling Point Elevation and Freezing Point Depression hotsell, The van t Hoff Factor Definition and How to Calculate It hotsell, If you had 1 L of water how many grams of NaCl will you need to hotsell, Solved the freezing depression formula Kf x m x i and NaCl has i hotsell, SOLVED Part A At what temperature would a 1.40 M NaCl solution hotsell, what mass of NaCl must be dissolved in 65 g of water to lower the hotsell, What is the freezing point of an aqueous solution containing 10.50 hotsell, SOLVED Part A At what temperature would a 1.40 M NaCl solution hotsell, 11.3 Colligative Properties General College Chemistry II hotsell, Solved COLLIGATIVE PROPERTIES. FREEZING POINT DEPRESSION Chegg hotsell, The van t Hoff Factor Definition and How to Calculate It hotsell, Freezing Point Depression Chemistry Steps hotsell, STEM Engine General Chemistry FREEZING POINT DEPRESSION hotsell, Van t Hoff Factor Definition Formula and Examples hotsell, Boiling Point Elevation and Freezing Point Depression hotsell, Answered EXPERIMENT 3 FREEZING POINT DEPRESSION bartleby hotsell, SOLVED Why does a 1.0m solution of AlCl3 have a lower freezing hotsell, ALEKS Calculating and using the van t Hoff factor for electrolytes 1 of 2 hotsell, Solved Observed Freezing Point F.Pt. and Freezing Point Chegg hotsell, 48. What mass of NaCl must be dissolved in 65.0 g of water to hotsell, What is Freezing Point Depression How it Works with Videos hotsell, 11.3 Colligative Properties General College Chemistry II hotsell, What is the van t Hoff factor for the NaCl in aqueous solution hotsell, Answered A. FREEZING POINT DEPRESSION Kf C m bartleby hotsell, The freezing point depression of 0.1 molal NaCl solution is 0.372 K. W hotsell, How would I solve for freezing point depression without molality hotsell, 0.5 mol of NaCl is dissolved in 500g of H2O. Then determine hotsell, The freezing point of a solution containing 5.85 g of NaCl in 100g hotsell, Assertion When NaCl is added to water a depression in Freezing hotsell, Water Free Full Text Investigation into Freezing Point hotsell, Calculate the freezing point of 0.1 M of a NaCl b glacial hotsell.
- freezing point depression vant hoff factor of nacl in water
- freezing temp given van't hoff factor
- freitag benjamin hoff
- friedan christina hoff sommers
- friederika hoff 1886
- friederika hoff 1886 17 children
- friedrich & christina hoff gohl
- friedrich & magdalena hoff
- friedrich adam hoff 1837
- friedrich adam hoff 1837 1913
-
Next Day Delivery by DPD
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.