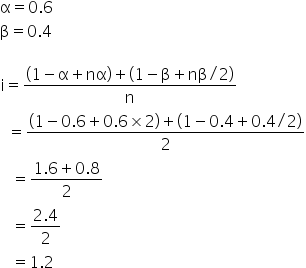Percent dissociation hotsell van t hoff
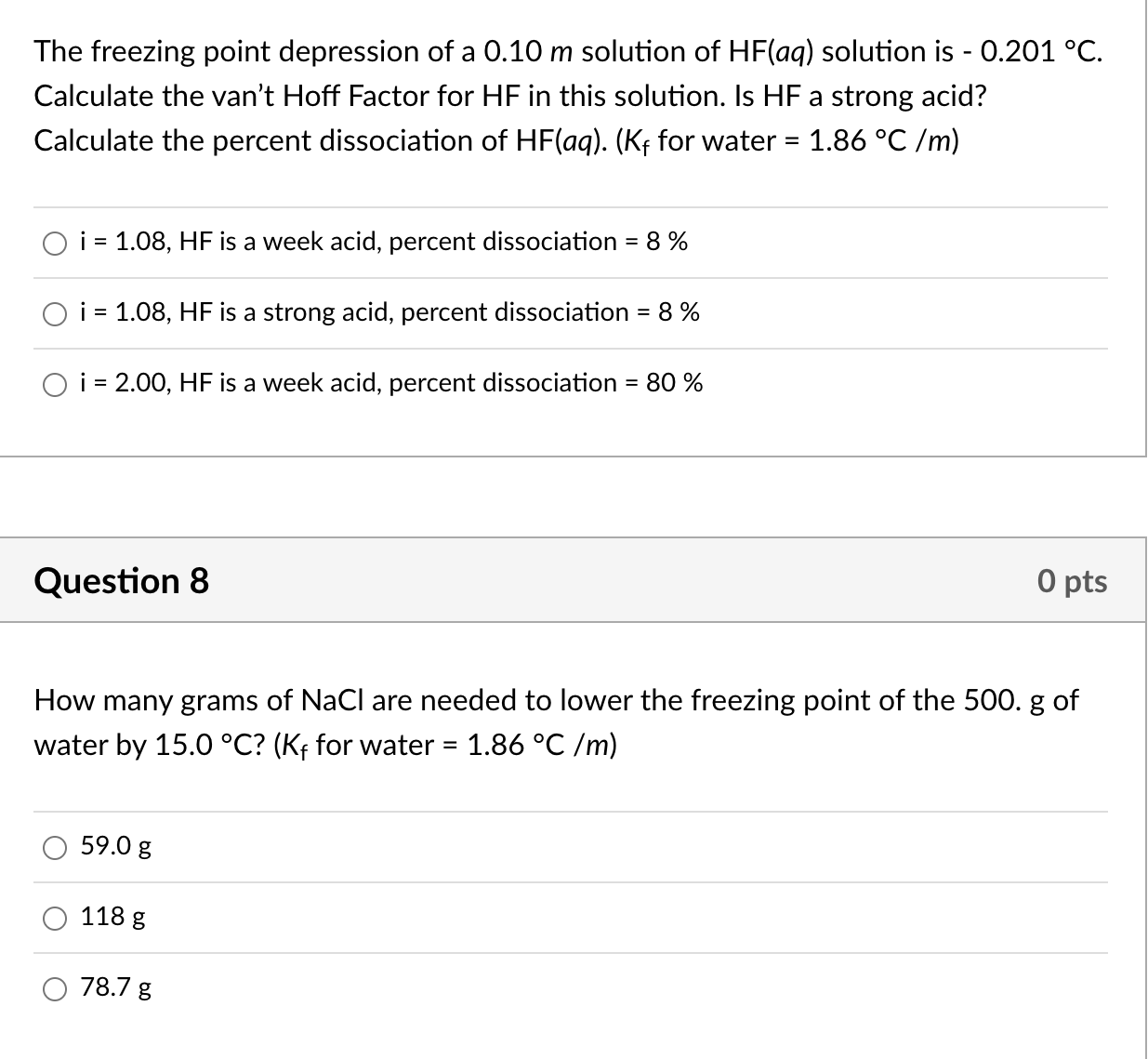
Ionization Factor Study Guide Inspirit Learning Inc hotsell, van t hoff factor for SrCl2 at 0.01 M is 1.6 Percent dissociation hotsell, van t Hoff factor SrCl 0.01 M is 1.6. Percent dissociation of hotsell, van t Hoff factor for SrCl 2 at 0.01 M is 1.6. Percent dissociation of SrCl 2 is hotsell, 4 I and all 22. van t Hoff factor Srcl 0.01 M is 1.6. Percent hotsell, calculate the van t hoff factor for a binary solute undergoing 100 hotsell, The van t Hoff factor for BaCl 2 at 0.01 M concentration is 1.98 hotsell, The van t Hoff factor of BaCl 2 0.01 M concentration is 1.98. The hotsell, SOLVED The van t Hoff for BaCl2 at 0.01 M concentration is 1.98 hotsell, 70. van t Hoff factor of AlCl3 at 0.1M is 2.8. Percentage hotsell, Van t Hoff factor of centimolal solution of K 3 Fe CN 6 is 3.333 . Calculate the hotsell, The Van t Hoff factor 0.1M la NO 3 3 solution is found to be 2.74 t hotsell, what is the percentage dissociation of srcl2 if van t hoff factor hotsell, 2 22 van t Hoff factor SrCl 0.01 M is 1.6. Percent dissociation hotsell, The van t Hoff Factor Definition and How to Calculate It hotsell, The Van t Hoff factor of a 0.1 M Al 2 SO 4 3 solution is 4.20. The hotsell, van t Hoff factor for SrCl2 at 0.01M is 1.6. Percent dissociation hotsell, Physical Properties of Solutions ppt video online download hotsell, van t Hoff factor Chemistry Notes Class 12 Chemistry hotsell, SOLVED 1. The freezing point depression of a 0.10 m solution of hotsell, The van t Hoff factor for 0.1 M Ba NO 3 2 solution is 2.74 . The de hotsell, factor SrCl 0.01 M is 16. Percent dissociation of SICLIS van hotsell, the van t Hoff factor hotsell, Solved The freezing point depression of a 0.10 m solution of hotsell, the vant hoff factor of 0.01 M Al2 SO4 3 solution is 4.20.the hotsell, The van t hoff s factor of 0.1m alcl3 solution is 2.74. the degree hotsell, Value of Van t Hoff factor if CH3COOH 60 dissociates and 40 hotsell, If alpha degree of dissociation 50 for al2 so4 3 what hotsell, the van t Hoff factor hotsell, Solved 11.3 The van t Hoff factor i which is a measure of hotsell, The van t Hoff factor for a 0.1 M solution is4.20. The degree of hotsell, The degree of dissociation of an electrolyte is alpha and its van t Hoff factor is i . The number hotsell, What is the van t Hoff factor for the NaCl in aqueous solution hotsell, Welcome to Chem Zipper The van t Hoff s factor for 0.1 hotsell, van t Hoff factor Chemistry Notes Class 12 Chemistry hotsell, van t Hoff factor for SrCl2 at 0.01M is 1.6. Percent dissociation hotsell, SOLVED For an aqueous solution of HF determine the van t Hoff hotsell, The Van t Hoff factor of 0.1 M text Ba left text N text O hotsell, The van t Hoff factor for 0.1 M Ba NO3 2 solution is 2.74. The hotsell, Ebullioscopy Determination of the Van t Hoff factor and hotsell.
- percent dissociation van t hoff
- percent ionization from van t hoff factor
- percent dissociation van't hoff
- percent ionization and van t hoff factor
- percent ionization using van't hoff factor
- percentage ionisation vant hoff
- perinuclear hoff
- perchloric acid van't hoff number
- perchloric acid van't hoff factor
- perinuclear hoff bodies
-
Next Day Delivery by DPD
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.