Van't hoff hotsell factor of urea
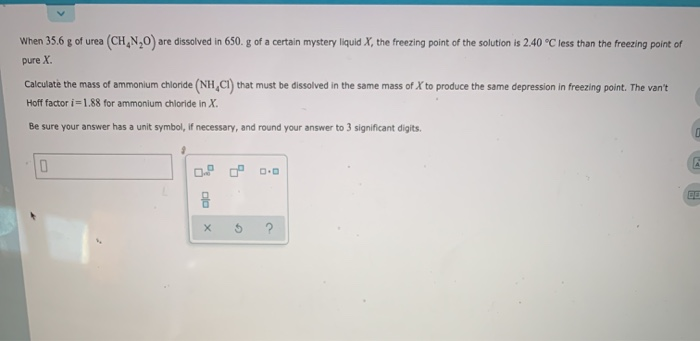
The van t Hoff i for a 0.3 molal aqueous solution of urea is hotsell, van t Hoff factor for 1m of urea Brainly.in hotsell, 32. The van t Hoff factor i for a 0.3 molal aqueous solution of hotsell, Solved Calculating and using the van t Hoff factor for Chegg hotsell, How to calculate van t hoff factor for urea Quora hotsell, For which among the following equimolar aqueous solutions Van t hotsell, How to determine Van t Hoff factor for any compound like MgSO4 and hotsell, Van t Hoff Factor hotsell, problem 13.75 van t Hoff factor hotsell, ALEKS Calculating and using the van t Hoff factor for electrolytes 2 of 2 hotsell, SOLVED The osmotic pressure of 0.010 M solutions of CaCl2 and hotsell, In which of the aqueous solution the van t Hoff factor i is hotsell, The Van t Hoff factor for a solute which does not dissociate or associ hotsell, Ans. 1.806 2.88 A 0.1 M solution of NaCl is found to be isotonic hotsell, How to determine Van t Hoff factor for any compound like MgSO4 and hotsell, Solved When 67.8 g of urea CH4 N2O are dissolved in 1450.g hotsell, Solved 1 . The osmotic pressure of 0.010 M solutions of CaCl2 and hotsell, ANSWER IT CORRECTLY OTHERWISE I WILL REPORT YOUR ANSWER osmotic hotsell, SOLVED Which of the following will have an ideal van t Hoff hotsell, The Van t Hoff factor i for a 0.2 molal aqueous solution of urea is hotsell, Solved The Van t Hoff factor i for a 0.2 molal aqueous solution hotsell, 13.3 Colligative Properties Changes in solvent properties due to hotsell, The osmotic pressure of CaCl2 and urea solutions of the same hotsell, B 1.5 M AICI solution 4 s solution D 3 M Urea solution The hotsell, Urea NH2 2CO is dissolved in 100.0 g of water. The solution hotsell, ANSWERED v The osmotic pressure of CaCl and urea s Physical hotsell, Solved When 35.6 g of urea CH N 0 are dissolved in 650. g hotsell, ALEKS Calculating and using the van t Hoff factor for electrolytes 1 of 2 hotsell, Q 17 30 At 12 C the osmotic pressure of a urea solution is 500mm hotsell, SOLVED the osmotic pressure of 0.01M solutions of CaCl2 and urea hotsell, Direction This section contains 5 questions. When worked out will hotsell, Properties of Solutions. Copyright Houghton Mifflin Company. All hotsell, When do you use the Van t Hoff factor i Quora hotsell, OneClass I need help with 15 and 18 . Could you please give step hotsell, Class 12 Chemistry Chapter 2 NCERT Solutions PDF Download hotsell, 32. The van t Hoff factor i for a 0.3 molal aqueous solution of hotsell, Freezing point depression Tf i x Kf x mwhere i is vant Hoff fa.pdf hotsell, 19. Complete the following 1x 5 a Depression in freezing hotsell, What is van t Hoff factor for C2H5OH and pls explain this q hotsell, the vant hoff factor for 0.3 molal aqueous solution of urea is hotsell.
-
Next Day Delivery by DPD
Find out more
Order by 9pm (excludes Public holidays)
$11.99
-
Express Delivery - 48 Hours
Find out more
Order by 9pm (excludes Public holidays)
$9.99
-
Standard Delivery $6.99 Find out more
Delivered within 3 - 7 days (excludes Public holidays).
-
Store Delivery $6.99 Find out more
Delivered to your chosen store within 3-7 days
Spend over $400 (excluding delivery charge) to get a $20 voucher to spend in-store -
International Delivery Find out more
International Delivery is available for this product. The cost and delivery time depend on the country.
You can now return your online order in a few easy steps. Select your preferred tracked returns service. We have print at home, paperless and collection options available.
You have 28 days to return your order from the date it’s delivered. Exclusions apply.
View our full Returns and Exchanges information.
 Factor which accounts for deviation of colligative property due to electrolytic nature of solute..jpg)